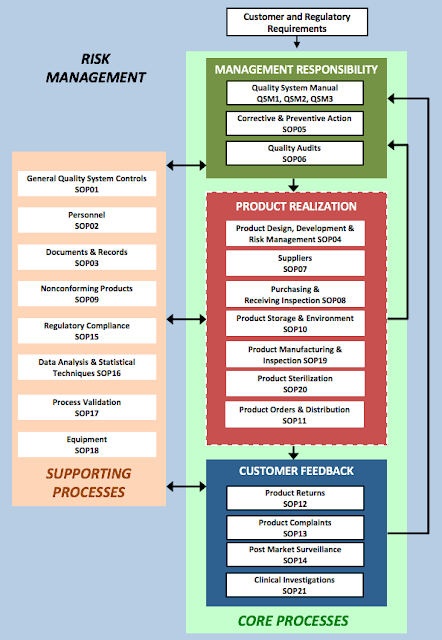
Sam Lazzara Resume LinkedIn Profile Documents Services Schedule Call +1 510 397 9739 sam@lazzara.net ISO 9000:2015 has replaced the 2000 version of the standard. This update has been timed to coincide with and support the ISO 9001:2015 published in September 2015. For us medical device folks, it is also helpful since it supports (EN) ISO 13485:2006 published in March 2016. Before you read the vocabulary definitions in ISO 9000:2015 you need to know what the "small words" mean. ISO's small word glossary is here . For example, did you know that "COMPLY" means a person or organization meet specified standards , whereas "CONFORM" means an ITEM meet specified requirements ? And according to ISO 9000:2015, an ITEM is an OBJECT, and an OBJECT is anything perceivable or conceivable (also known as an ENTITY). And examples of ITEMs are p roduct, service, process, person, organization, system, and resource. To understand the difference